Zynex Receives FDA Clearance for Next-Gen M-Wave Neuromuscular Stimulation Device
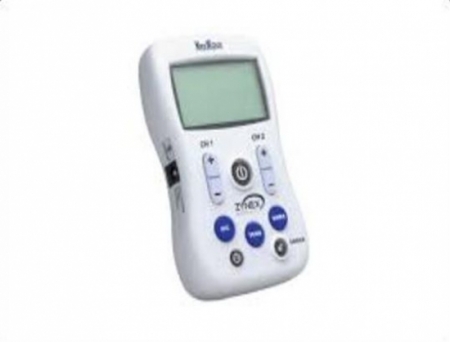
Zynex, Inc., a medical technology firm, has announced FDA clearance for its advanced M-Wave Neuromuscular Electrical Stimulation (NMES) device, marking a significant milestone in the field of non-invasive medical technology.
The M-Wave, the successor to the renowned E-Wave, revolutionizes NMES treatments with enhanced functionality and a user-friendly design. With over two decades of expertise behind it, the E-Wave has been instrumental in NMES therapy across the United States since 1998. Now, the M-Wave builds upon this legacy, offering clinicians and patients alike a more intuitive and versatile solution.
Thomas Sandgaard, CEO of Zynex Medical, emphasized the M-Wave's evolution as a response to user feedback, ensuring a more tailored and effective therapeutic experience. "The M-Wave introduces the next evolution in NMES devices, allowing for more customizable treatments within clinical and home settings," said Sandgaard. "Our Product Management team has incorporated patient and physician feedback when designing the new M-Wave."
The M-Wave's advanced features and user-friendly interface aim to improve neuromuscular condition management for patients. Its compact and lightweight design ensures portability and easy integration into both clinical and home environments, facilitating swift and effective treatment.
This FDA clearance underscores Zynex's commitment to innovation in medical technology, offering patients and healthcare professionals cutting-edge solutions for pain management, rehabilitation, and patient monitoring.
Last news about this category
We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy

















