Biolinq Secures FDA De Novo Classification for Wearable Glucose Sensor
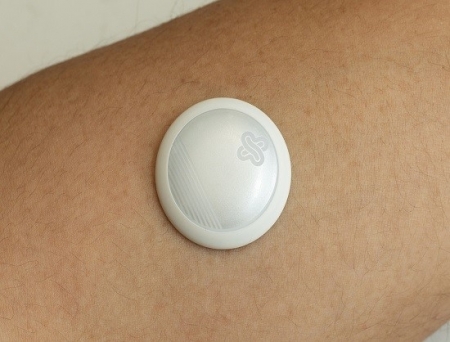
San Diego-based health tech startup Biolinq Incorporated has announced that the US Food and Drug Administration (FDA) has granted De Novo Classification for its lead product, Biolinq Shine, a fully autonomous, needle-free glucose sensor.
This milestone paves the way for Biolinq to scale a new generation of wearable sensors designed for comfort, simplicity, and global reach.
Initially aimed at people with type 2 diabetes who are not dependent on insulin, Biolinq Shine is the first wearable biosensor integrating glucose, activity, and sleep information in a single device with autonomous operation. Worn as a patch on the forearm, it provides real-time glucose feedback through a primary colour-coded LED display—readable with or without a smartphone—while additional insights such as activity and sleep trends, are available through a mobile app.
Unlike traditional filament-based glucose sensors, Biolinq Shine eliminates the need for a subcutaneous introducer needle. Its microsensor array, manufactured using state-of-the-art semiconductor technology, penetrates up to 20 times more shallowly than conventional continuous glucose sensors.
“While we celebrate this tremendous milestone, we’ve only scratched the surface of what is possible with our multi-analyte-capable biosensor platform in supporting metabolic health for everyone,” said Rich Yang, CEO of Biolinq.
Dan Bradbury, Chairman of Biolinq, noted that Biolinq Shine is a first-of-its kind biosensor designed to support metabolic health for people with diabetes who are not dependent on insulin.
“By automatically tracking glucose levels, physical activity and sleep information, this technology offers meaningful insights that can encourage healthier choices every day,” he added.
, Biolinq Shine Biolinq Incorporated De Novo Classification Needle-free glucose sensor US Food and Drug Administration (FDA) Wearable Glucose Sensor
Last news about this category
We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy
















