FX Shoulder Solutions Secures FDA Clearance for Full-Wedge Augmented Glenoid Baseplates
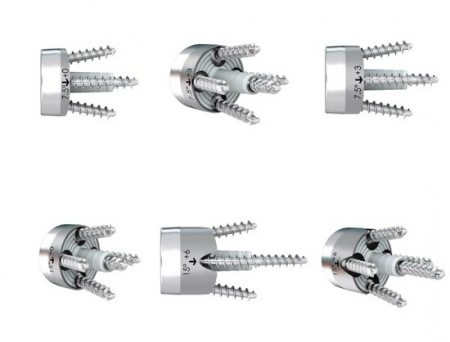
FX Shoulder Solutions, Inc. has achieved FDA 510k clearance for its full-wedge augmented glenoid baseplates, introducing six new options to its existing portfolio.
This clearance brings the total number of glenoid baseplate options to 18, providing a variety of solutions for surgeons addressing bone loss, defects, or complicated glenoid morphologies.
The newly cleared glenoid baseplates are all 24mm in diameter and come in full-wedge options at 7.5° and 15°, with lateralization options of 0, +3, or +6mm. Each baseplate features four peripheral screw holes with 12° of polyaxial variability, allowing for fixation with 4.5mm standard or locking screws. Additionally, a 4.5mm central screw can be utilized through the central post, available in seven length options from 8-20mm in 2mm increments. All baseplates are constructed from titanium (Ti6AV) and coated with hydroxyapatite (CP Ti/HAP).
"This clearance of the full-wedge augmented baseplates further solidifies our portfolio as one of the most comprehensive and innovative shoulder arthroplasty platforms on the market," stated Baptiste Martin, CEO of FX Shoulder Solutions.
FX Shoulder Solutions, headquartered in Addison, TX, continues to innovate based on surgeon feedback and market trends, with a dedicated focus on shoulder arthroplasty.
Baptiste Martin Bone loss FDA 510k clearance Full-Wedge Augmented Glenoid Baseplates FX Shoulder Solutions Glenoid morphologies
Last news about this category
We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy
















