Integral Molecular Launches Ready Reporter Virus Kit for 10x Faster Virus Neutralisation Testing
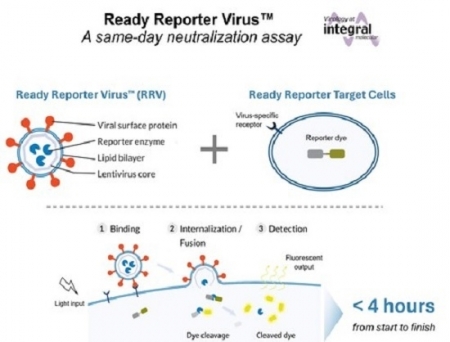
Integral Molecular has launched its Ready Reporter Virus (RRV) neutralisation assay kit. This automation-compatible, safe assay assesses the effectiveness of vaccines and therapeutic antibodies and is set to transform how virus neutralisation testing is performed. While conventional assays can take up to a week, the RRV kit delivers results in under four hours—10 times faster than current standards.
RRVs work by instantaneous detection of viral entry using a fluorescence-based readout. This rapid, safe and simple assay enables automatable workflows that can be performed in a standard laboratory setting.
Conventional neutralisation assays are labour intensive, requiring live pathogenic virus, specialised biosafety facilities, cultivation of cells under sterile conditions, and often manual counting of results. These barriers make testing impossible for most laboratories worldwide. In contrast, RRVs do not replicate or cause disease and do not require cell culture, making this assay accessible to all scientists. RRV kits come with thaw-and-mix reagents and use simple workflows ideal for automation, a key consideration for scientists who need to screen hundreds or even thousands of samples.
The RRV kit can be used for a wide range of applications, including serum testing from humans or animals to assess vaccine-induced neutralisation, neutralisation testing and lot-release for therapeutic antibodies, as well as public health initiatives such as disease surveillance.
"Integral Molecular has long been committed to delivering safe, reliable tools that expand the ability of labs to conduct antiviral research critical for vaccine development. With RRVs, we are making neutralisation testing not just faster, but also simpler, cost-effective, and more accessible for scientists who need robust, automatable assays without the burden of cell culture," said Benjamin Doranz, PhD, CEO, Integral Molecular.
RRV kits are currently available for viruses including influenza, SARS-CoV-2, and VSV. Kits are manufactured under ISO 9001-certified quality processes and can be customised for additional viruses or variants of interest. RRVs will be launched in a presentation by Christina Go, PhD, at the World Vaccine Congress this week in Amsterdam.
Automatable workflows Benjamin Doranz Integral Molecular Ready Reporter Virus Kit Serum testing Therapeutic antibodies Virus Neutralisation Testing World Vaccine Congress
Last news about this category
We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy

















