ManaMed Secures FDA Clearance for Next-Generation Tubeless Compression System, PlasmaFlow X
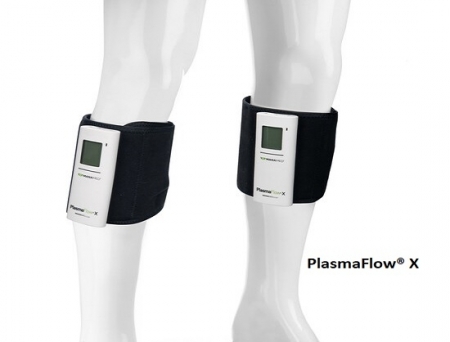
ManaMed, a US-based global leader in orthopedic mobility and post-operative rehabilitation equipment, has received FDA 510(k) clearance for PlasmaFlow X, its next-generation tubeless sequential compression system designed to redefine recovery and enhance patient comfort.
Cleared as a Class II medical device, PlasmaFlow X introduces a more compact, smarter, and longer-lasting design that delivers powerful therapy in a sleek, portable package. The device is engineered to aid in deep vein thrombosis (DVT) prevention, reduce post-operative pain and swelling, and enhance blood circulation in patients who are stationary for extended periods.
The compact design helps save shelf space in surgery centers and warehouses nationwide—while reducing packaging waste. Built for both clinical and home use, PlasmaFlow X features an all-day battery offering double the life of the previous model, a USB-C charging system, and an expanded LCD display that provides real-time updates on battery level, compression pressure (mmHg), and patient adherence. Its low-profile, lightweight sleeve ensures comfort and mobility, while its tubeless configuration simplifies therapy delivery in clinical and home environments.
Trevor Theriot, President + CEO of ManaMed, said, "PlasmaFlow X is a generational leap for sequential compression. In a category long defined by incremental updates, our team re–imagined everything—a more compact design, all–day battery, USB–C convenience, and a big beautiful, informative display—to deliver industry leading therapy in a remarkably portable system. This is our most exciting product launch in ManaMed's 10–year history, and it underscores our commitment to innovation that serves patients, clinicians, and partners."
With FDA clearance secured, PlasmaFlow X is now approved for marketing in the United States and will be available through ManaMed and its authorised distributors.
Deep vein thrombosis (DVT) prevention FDA 510(k) clearance ManaMed PlasmaFlow X Post-operative pain Post-operative rehabilitation Tubeless sequential compression system
Last news about this category
We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy
















