OrtoWell Hydraulic Distractor System Proven Safe and Effective in First Multi-Centre Clinical Study
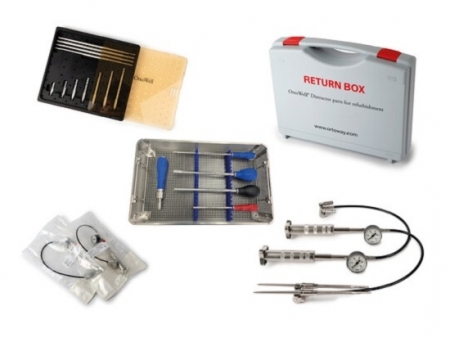
OrtoWay-US Inc, the US-German MedTech innovator focused on spinal surgery tools for orthopedic vertebral separation, has announced the publication of the first prospective, multi-center clinical study evaluating its OrtoWell Distractor System – the world's first hydraulically powered vertebral distractor.
Published in Medical Devices: Evidence and Research (Dove Medical Press, October 2025) and available in abstract on PubMed, the study confirms the safety, usability and intraoperative performance of the device across 30 patients treated in Germany. The results showed no device-related complications and strong surgeon feedback on the system’s precision, stability and control.
Unlike conventional instruments such as mallets or torque-based retractors, the OrtoWell Distractor uses a hydraulic mechanism that enables surgeons to open the vertebrae smoothly and safely, with finely controlled incremental pressure, reducing the risk of over-distraction, neural damage and other complications.
"The data confirms what surgeons have long reported – that hydraulic distraction offers a gentler, more controllable approach to vertebral separation than mechanical systems. The OrtoWell Distractor gives surgeons a new level of tactile precision, helping reduce procedural risk while improving predictability in spinal reconstruction. This is a key step to establishing a new future standard in spinal surgery,” said Stan Mikulowski, CEO of OrtoWay-US Inc.
Conducted between 2018 and 2023 at three German independent spine centres in Bonn, Essen and Tutzing, and led by Professor Robert Pflugmacher of the University of Bonn, the study demonstrated that controlled hydraulic distraction significantly improves alignment and fixation during surgery.
Across all 30 cases, the OrtoWell system simplified complex spinal procedures, provided stable intraoperative parameters, and reduced the risk of cortical wall damage or retractor slippage — complications that can otherwise necessitate spinal fusion.
Manufactured in Tuttlingen, Germany, the OrtoWell Distractor System is approved for use in the United States for all prosthesis types.
The latest findings build on earlier work led by Dr. Biren Desai at Dreifaltigkeits-Krankenhaus Clinic in Cologne, which first demonstrated successful multi-level corpectomy using the OrtoWell system.
"Hydraulic control not only reduces the risk of vertebral damage — it transforms the surgical workflow. We see this as the start of a broader shift toward more ergonomic, precision-based surgical tools,” added Mikulowski.
Orthopedic vertebral separation OrtoWell Hydraulic Distractor System Spinal surgery tools Stan Mikulowski, CEO of OrtoWay-US
Last news about this category

We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy
















