PENTAX Medical Sells C2 CryoBalloon Technology to Merit Medical Systems
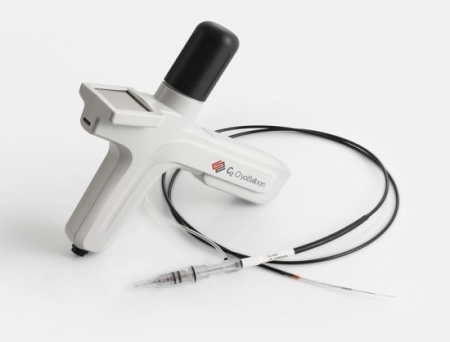
PENTAX Medical, a division of the Japanese med-tech company HOYA Group and a global leader in flexible reusable endoscopy, has signed an asset purchase agreement with the American healthcare technology company Merit Medical Systems for the sale of its C2 CryoBalloon technology, a minimally invasive solution for treating Barrett’s Esophagus and other gastrointestinal disorders.
The company stated that this strategic move will enable it to sharpen its focus on core endoscopic solutions while fostering deeper clinical partnerships.
The C2 CryoBalloon, which has been part of PENTAX Medical's therapeutic portfolio since 2017, delivers controlled cryotherapy to ablate unwanted tissue while preserving surrounding structures. Under Merit Medical's stewardship, the technology is expected to reach new levels of adoption and impact, expanding access to care for patients suffering from chronic GERD and related conditions.
Dominique Vincent, President of PENTAX Medical, said, "C2 technology has been an exciting part of our innovation journey. We are proud of the clinical value it has delivered and are confident that Merit Medical, with its expanding footprint in upper GI treatments and deep expertise in therapeutic endoscopy, is ideally positioned to unlock the full potential of C2.”
“This transition allows PENTAX Medical to refocus on our core strength—flexible reusable endoscopy—and continue delivering intuitive solutions that empower clinicians worldwide,” Vincent added.
Merit Medical will integrate the C2 CryoBalloon into its Endoscopy portfolio, complementing its existing products and customer base.
Over the coming months, product manufacturing will be transferred to Merit’s facility in South Jordan, Utah, with several PENTAX Medical employees joining the company, bringing valuable expertise and continuity to the team.
Barrett’s Esophagus C2 CryoBalloon technology Chronic GERD Gastrointestinal disorders HOYA Group Merit Medical Systems PENTAX Medical Reusable endoscopy
Last news about this category

We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy
















