Sun Pharma's Partner Philogen Reports Promising Trends from Phase III Fibromun Trial in Soft Tissue Sarcoma
Sun Pharmaceutical’s partner Philogen, a Swiss-Italian biotechnology company, has provided an update on the Phase III FIBROSARC trial evaluating Fibromun (L19TNF) in combination with doxorubicin versus doxorubicin alone as first-line treatment for patients with advanced or metastatic Soft Tissue Sarcoma (STS).
STS represent a heterogeneous group of more than 100 tumour subtypes originating from mesenchymal or connective tissues and account for about one percent of all adult cancers. Doxorubicin monotherapy remains the current standard first-line therapy for most subtypes of advanced or metastatic disease.
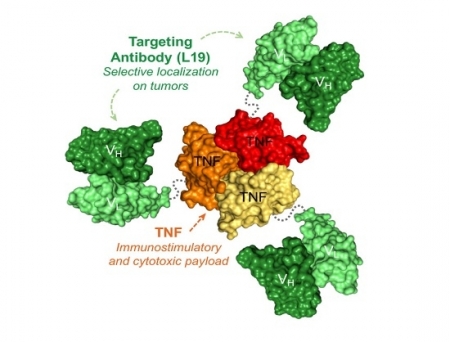
Fibromun is a fully-human immunomodulatory fusion protein, combining the L19 antibody—which targets the EDB domain of fibronectin—with TNF (tumour necrosis factor), a strong pro-inflammatory cytokine. This combination results in a tumour-targeted product, which preferentially localises at the site of disease, while sparing healthy organs. In several immunocompetent preclinical models, Fibromun has shown potent anti-tumour activity, both as a single agent and in combination with other drugs, inducing long-lasting complete responses in most cases.
In the FIBROSARC Phase III study, patients with leiomyosarcoma, liposarcoma and other rare histologies were enrolled. The primary endpoint was Progression-Free Survival (PFS), and Overall Survival (OS) was a secondary endpoint. Although the trial did not meet its primary PFS endpoint, results showed promising trends across key efficacy parameters—PFS, Objective Response Rate (ORR) and OS—favouring the Fibromun plus doxorubicin arm over doxorubicin alone.
Philogen plans to present full results at upcoming scientific conferences and submit findings for publication in a peer-reviewed journal in 2026. Encouraged by the trends and the significant unmet need in STS, the company intends to discuss the data with European and US regulators and initiate a confirmatory Phase III trial focused on OS as the primary endpoint in 2026.
Professor Christoph Schliemann of the University Hospital Münster, study coordinator and principal investigator, commented, “Fibromun is showing signs of activity in a very difficult-to-treat indication. We remain interested in future applications of this investigational study drug. We are eager to learn about the evolution of overall survival data.”
Fibromun has been granted orphan drug designation for the treatment of STS by both the European Commission and the US Food and Drug Administration.
Philogen also shared updates on other ongoing STS programmes: FLASH and FIBROSARC US.
FLASH: It is a randomised controlled Phase II trial evaluating evaluating Fibromun with dacarbazine as last-line therapy in advanced or metastatic STS. Patient enrolment has been completed, and the final PFS readout is expected by end of 2025.
FIBROSARC US: It is a randomised controlled Phase IIb trial assessing Fibromun with doxorubicin as first-line treatment in metastatic leiomyosarcoma. So far, 81 of 158 patients have been enrolled. An interim analysis at 50 percent PFS events is planned for Q1 2026, to be reviewed by an independent Data Safety Monitoring Board (DSMB).
Doxorubicin monotherapy Fibromun (L19TNF) Phase III FIBROSARC trial Philogen Soft Tissue Sarcoma (STS) Sun Pharmaceutical
Last news about this category
We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy

















