Xenetic Biosciences, Scripps Research Extend Collaboration to Advance DNase I and CAR-T Combination Programme
Xenetic Biosciences has extended its research collaboration with The Scripps Research Institute and the laboratory of Dr Alexey Stepanov, Institute Investigator at Scripps Research, for an additional four months, effective 1 November 2025. The partnership aims to advance the company’s R&D programme, evaluating the combined use of systemic DNase I and CAR T-cell therapies for difficult-to-treat cancers.
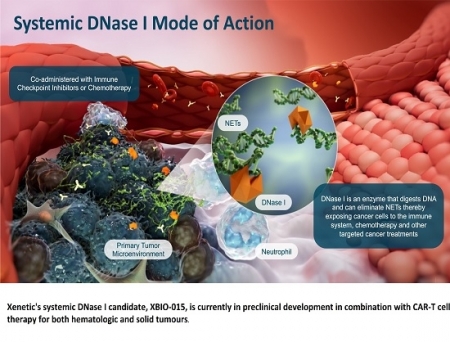
Xenetic’s systemic DNase I candidate, XBIO-015, is in preclinical development alongside CAR-T therapies for both haematologic and solid tumours. Preclinical studies conducted by Dr Stepanov’s team in lymphoma, metastatic melanoma and leukaemia models have shown that co-administration of DNase I with CAR-T cells significantly reduces tumour burden, decreases metastatic lesions, and markedly extends survival compared to CAR-T cell monotherapy.
The company explains that systemic DNase I-mediated degradation of neutrophil extracellular traps (NETs) enhances CAR-T cell efficacy, increasing the infiltration of both CAR-T cells and endogenous T cells into tumours and mitigating the immunosuppressive tumour microenvironment (TME).
James Parslow, Interim CEO and CFO of Xenetic, said the extended partnership underscores the promise of the company’s DNase-based oncology platform.
"The expertise and dedication of the Scripps Research team to this programme further validates our belief in DNase I to improve therapeutic responses in patients undergoing CAR-T cell therapy and we look forward to continued collaboration and innovation together,” he added.
Xenetic is progressing its DNase-based technology towards Phase 1 clinical development for the treatment of pancreatic carcinoma and other locally advanced or metastatic solid tumours. Preclinical proof-of-concept studies combining DNase I with chemotherapy, immunotherapies and CAR-T therapy in hematological and solid tumour and metastatic cancer models have been completed. Building on proof-of-concept success, the programme has now advanced to mechanism-of-action and translational studies in preparation for a Phase 1 clinical trial.
Xenetic Biosciences DNase-based oncology platform Dr Alexey Stepanov, Institute Investigator at Scri Metastatic melanoma and leukaemia Systemic DNase I and CAR T-cell therapies The Scripps Research Institute Xenetic’s systemic DNase I candidate XBIO-015
Last news about this category

We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy
















