Zimmer Biomet Receives FDA Clearance for Enhanced ROSA Knee with OptimiZe System
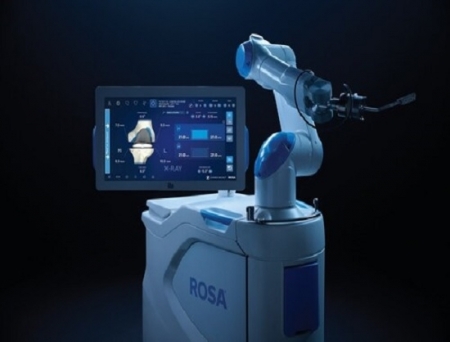
Zimmer Biomet Holdings, a global leader in medical technology, has received US Food and Drug Administration (FDA) 510(k) clearance of its ROSA Knee with OptimiZe, an enhanced version of its ROSA Knee System designed to deliver greater personalisation, accuracy and reproducibility in robotic-assisted total knee replacement surgery.
The new ROSA Knee with OptimiZe introduces a more customised experience for surgeons through intelligent surgical planning and advanced positioning, tracking and alignment features.
The technology provides a simplified user interface that allows surgeons to choose the information they want to see when they need it.
Designed for use with the industry-leading Persona Knee System, ROSA Knee with OptimiZe enables surgeons who use functional alignment to use customisable surgeon profiles that systematically provide surgical plans to automatically position the implant and balance the knee based on the patient's own anatomy and the surgeon's preferences. For surgeons who prefer kinematic alignment, the system offers the industry's only automated kinematic alignment feature to resurface the knee with the goal of restoring its pre-arthritic position and native joint lines.
"As a leader in advancing innovation in orthopedic robotics, we are committed to improving and enhancing our robotic technologies to better meet the needs of surgeons and improve efficiency in the OR, with the ultimate goal of delivering better outcomes for patients," said Shaun Braun, senior vice president and chief information and technology officer at Zimmer Biomet.
"ROSA Knee with OptimiZe was designed in partnership with our seasoned team of ROSA surgeons to make robotic-assisted knee replacement surgery more personalised, accurate and efficient for surgeons. With our proprietary algorithm, OptimiZe Planning, surgeons can create customised profiles that generate personalised surgical plans, which can reduce planning time by an average of 46 percent,” he added.
ROSA Knee with OptimiZe also integrates with ZBEdge Analytics, allowing surgeons to make data-driven intra-operative decisions, objectively assess their performance and understand the potential impact of clinical decisions on patient recovery.
Zimmer Biomet plans a targeted release of ROSA Knee with OptimiZe later this year, with commercial availability in the US expected in the first quarter of 2026.
Zimmer Biomet Medical technology Robotic-assisted total knee replacement surgery ROSA Knee System ROSA Knee with OptimiZe US Food and Drug Administration (FDA) 510(k) clear
Last news about this category
We use our own and third party cookies to produce statistical information and show you personalized advertising by analyzing your browsing, according to our COOKIES POLICY. If you continue visiting our Site, you accept its use.
More information: Privacy Policy
















